A Unique in vitro Model
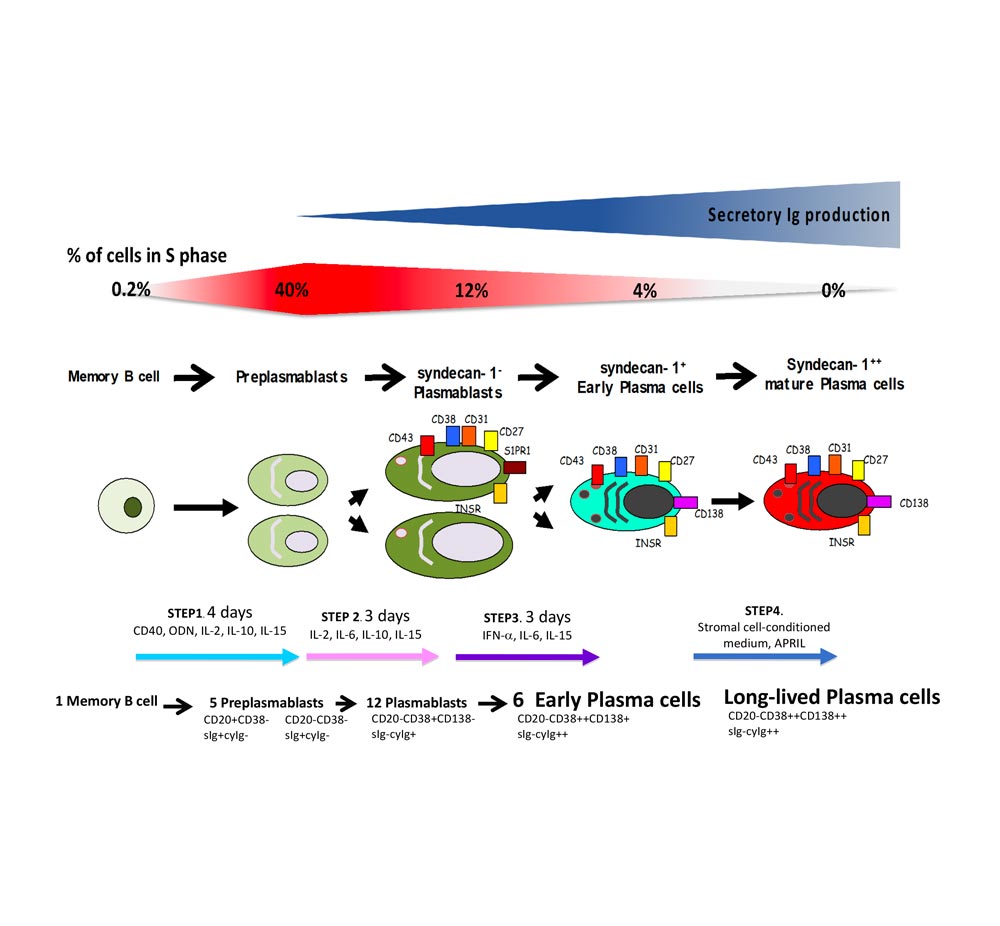
The differentiation of B cells to plasma cells (PCs) is essential for humoral immunity and protect the host against infections. In human, PC are rare cells with differentiation stages taking place in anatomic places that hamper full biological characterization. To study the coordinated transcriptional changes and the phenotype of the different B to PC stages that can be detected in vivo, our partner lab has developed an in vitro B to PC differentiation model using multi-step culture systems. To reproduce the sequential cell differentiation occurring in the different organs/tissues in vivo, we use various combinations of activation molecules and cytokines for the different steps.
In the first step, memory B cells are first activated for four days by CD40 ligand, oligodeoxynucleotides and cytokine combination and differentiate into pre-plasmablasts (PrePBs). In the second step, pre-plasmablasts are induced to differentiate into plasmablasts (PBs) by removing CD40L and oligodeoxynucleotides stimulation and changing the cytokine combination. In the third step, plasmablasts are induced to differentiate into early PCs by changing the cytokine combination. The fourth step allows getting fully mature PCs by culturing these early PCs with bone marrow stromal cells or selected growth factors. These mature PCs could survive several months in vitro and secrete high amounts of immunoglobulin.
The Characterization of the Model
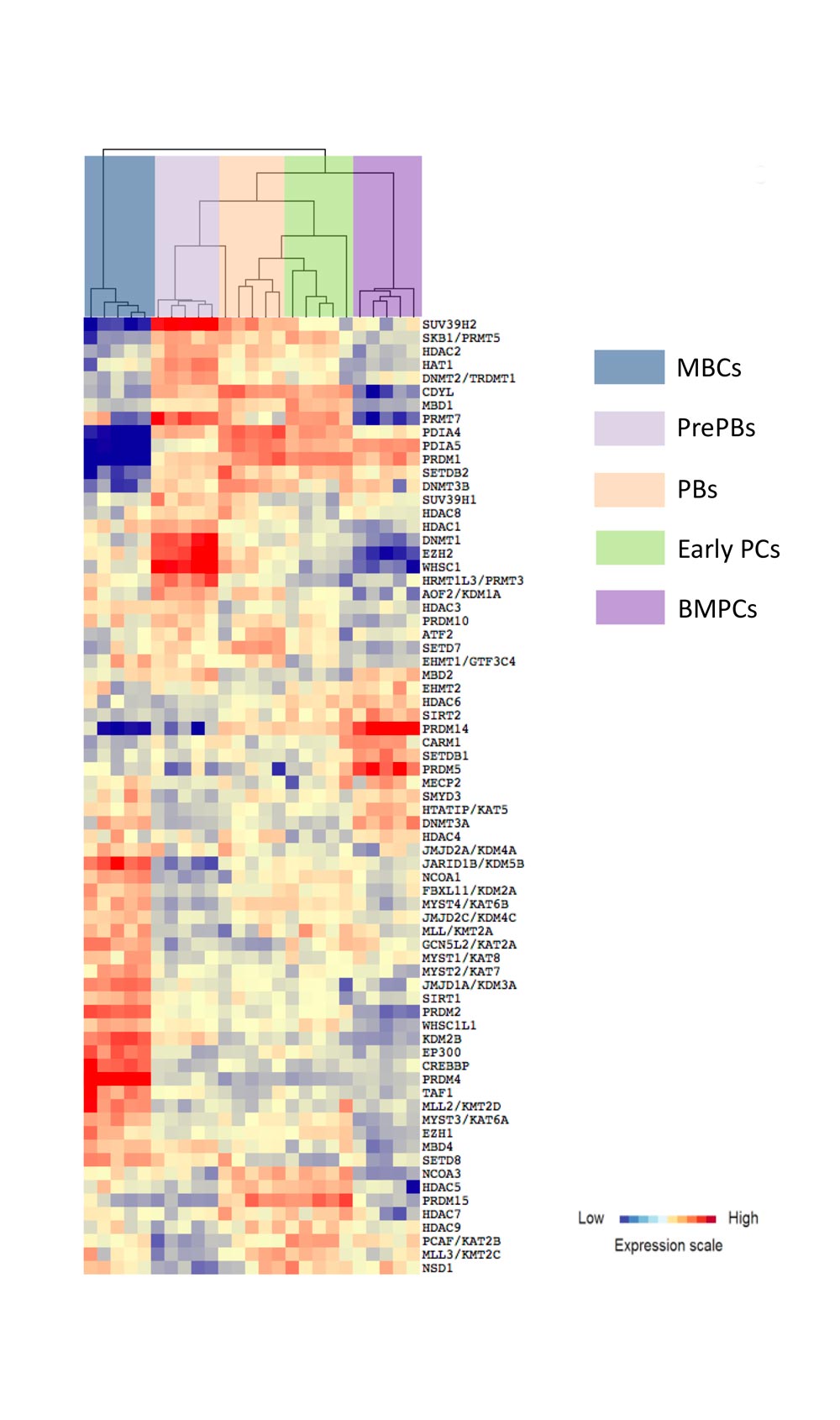
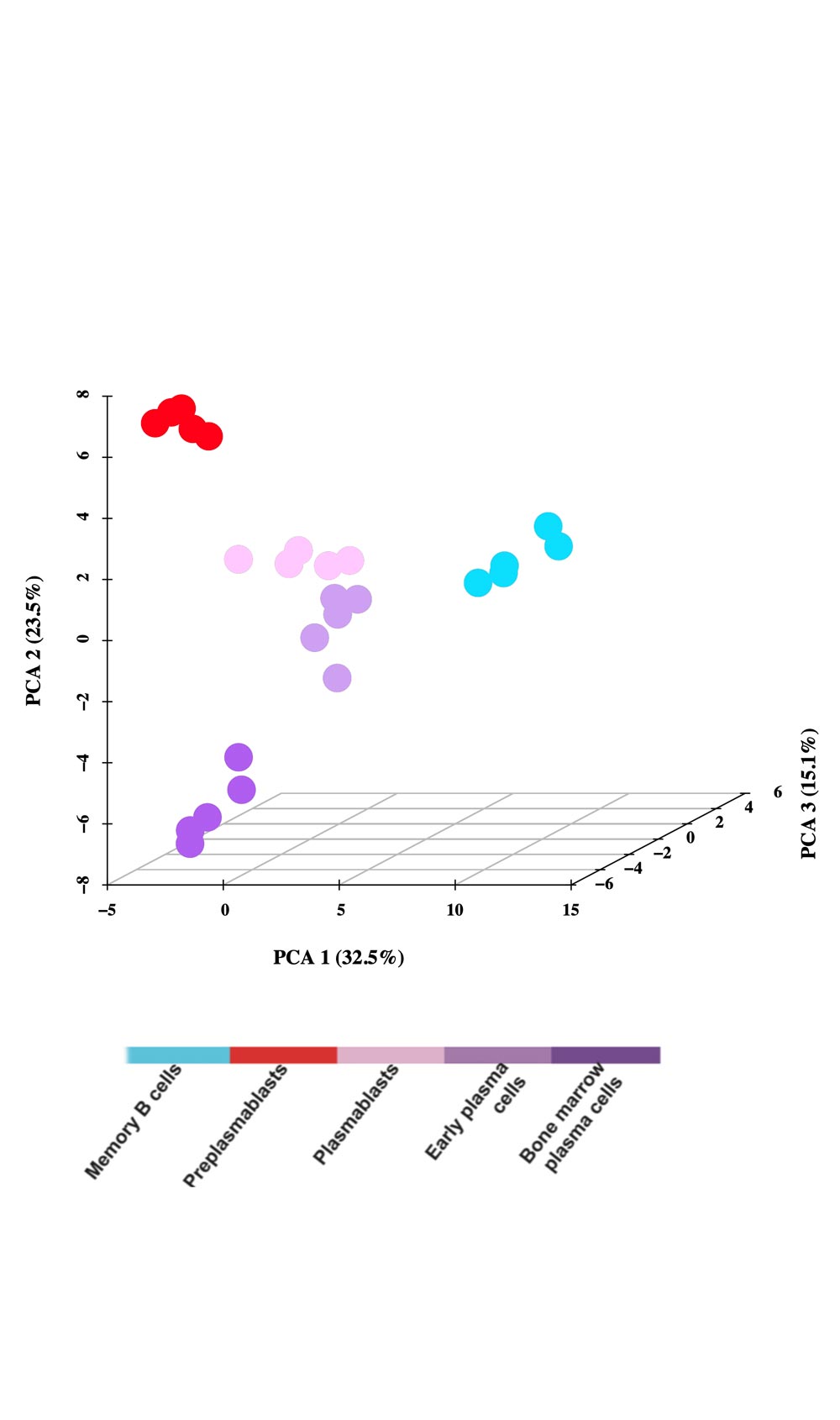
A moderate heterogeneity could be observed in the percentage of activated B cells, PrePBs, PBs and, PCs depending on healthy donor’s blood used for memory B cell purification. The generated long-lived PCs are non-cycling PCs, surviving and producing immunoglobulins for more than three months in vitro, as their in vivo counterpart. Long-lived PCs express highly CD138 and gene expression profiles related to in vivo PCs. Using the gene expression profiling of cell subpopulations, the expression changes of the epigenetic factors have investigated. Additionally, the miRnome of normal PC differentiation has also performed and identified novel key miRNAs regulating networks of significance for normal PC differentiation.
- The knowledge derived from this research model could inform and instruct on diagnostic and therapeutic strategies for PC disorders, such as in the case of multiple myeloma.
- We offer you the investigation of given drug effects on this normal human plasma cell generation using this unique in vitro model. Recently, our partner lab published a study (see here) realized with Celgene to investigate differential effects of lenalidomide during plasma cell differentiation.